
AIR OPTIXTM plus HydraGlydeTM for Astigmatism monthly contact lenses give your consumers outstanding comfort – just like wearing their favourite pair of jeans.
Help new contact lens wearers avoid dryness and discomfort
Eyeglass only wearers who used to wear contact lenses11
- Non-astigmatic Patients 16%
- Non-astigmatic Patients 32%
Common reasons for astigmatic consumers to drop out of contact lens wear:12

Dryness

Discomfort

Poor vision
Help your new astigmatic consumers enjoy stable lens wear,9‡ deposit resistance1,2** and comfort from day 1 to day 301-4** with AIR OPTIXTM plus HydraGlydeTM for Astigmatism contact lenses.
SUPERIOR DEPOSIT RESISTANCE1,2* FOR CONSISTENT COMFORT1-4**
Advanced SmartShield™ Technology keeps the contact lens clean
and comfortable throughout the wearing period.1-4**
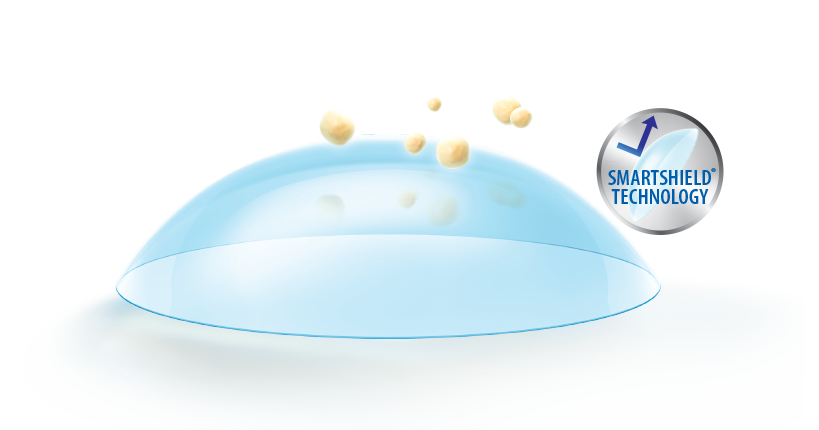
SmartShield™ Technology enables comfort from day 1 to day 301-4** by creating a barrier against:

Lipid deposits1,2**

Cosmetic creams5†

Mascara5†
Combining AIR OPTIXTM plus HydraGlydeTM contact lenses with HydraGlydeTM lens care solutions enables less cholesterol buildup than competing brands.6

KEEP YOUR ASTIGMATIC CONSUMERS HAPPY WITH HYDRAGLYDETM MOISTURE MATRIX
In addition to SmartShield™ Technology, AIR OPTIXTM plus HydraGlydeTM for Astigmatism contact lenses feature the HydraGlydeTM Moisture Matrix for longer-lasting moisture.7,8††

How Does HydraGlydeTM Moisture Matrix Work?
- The wetting agent is absorbed by the lens surface.
- The HydraGlydeTM Moisture Matrix interacts with SmartShield™ Technology, creating a hydrophilic environment to help attract and retain moisture.7
- This results in an envelope of longer-lasting moisture at the lens surface.7,8††
SUCCESSFUL LENS WEAR FOR ASTIGMATIC CONSUMERS
AIR OPTIXTM plus HydraGlydeTM for Astigmatism contact lenses minimize oscillation and provide the on-eye stability astigmatic consumers need.9‡
The Precision Balance 8|4™ Lens Design
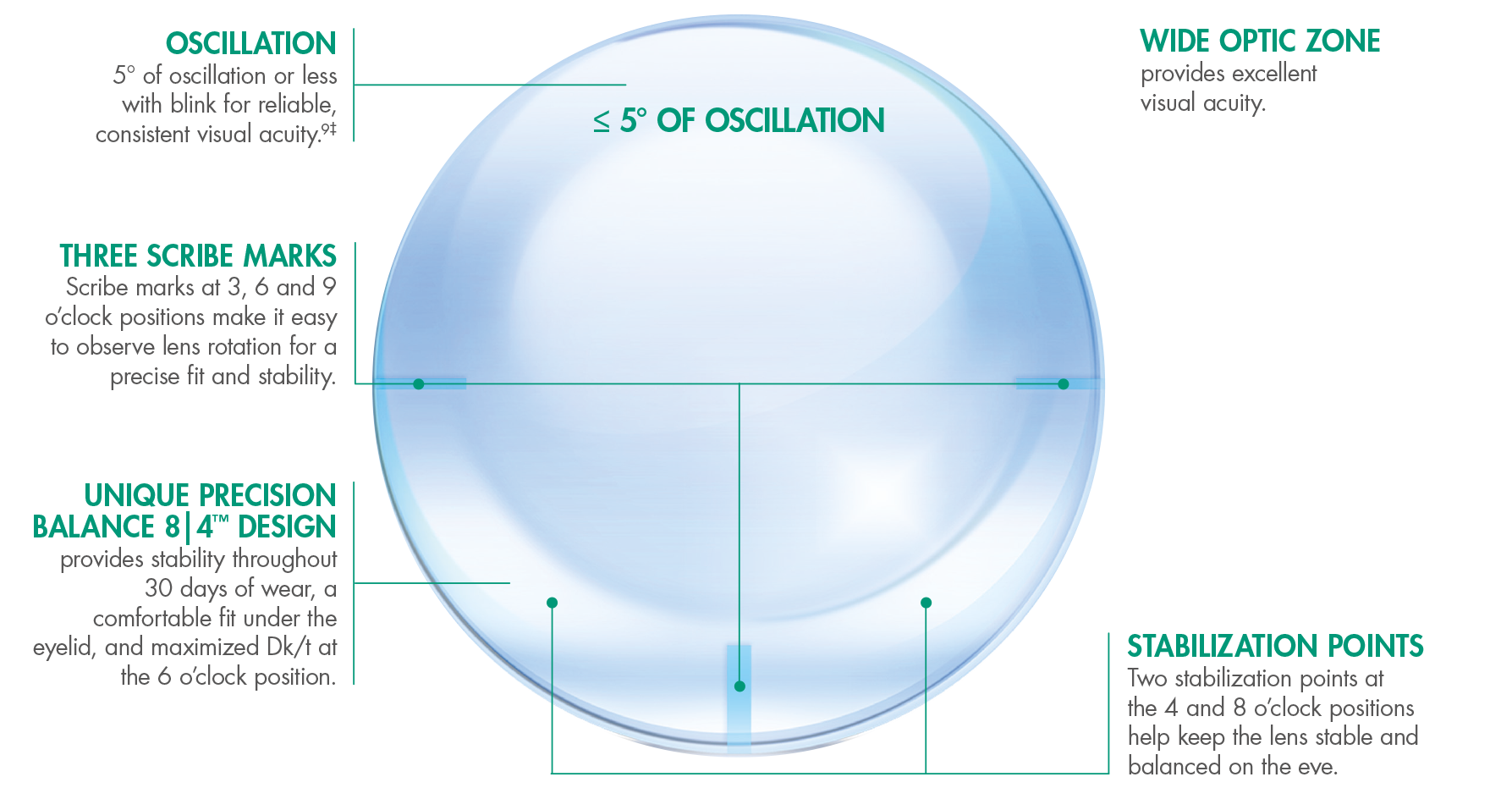
Wide Coverage for Astigmatic Patients
-
Coverage for 98.9% of astigmatic eyes‡‡ with a spectacle cylinder of up to -3.00DC10
-
Coverage for 94.7% of all astigmatic eyes10‡‡
Offer AIR OPTIXTM plus HydraGlydeTM for Astigmatism contact lenses
for a successful first-time contact lens-wearing experience.
Give Your Astigmatic Consumers The HydraGlydeTM Advantage
Combine AIR OPTIXTM plus HydraGlydeTM for Astigmatism contact lenses with a HydraGlydeTM lens care solution for even more comfortable wear time.

3 MORE hours
OF COMFORTABLE WEAR TIME9-11††,‡ with Alcon’s hydrogen peroxide solution.

2 MORE hours
OF COMFORTABLE WEAR TIME9-11††,‡ with Alcon’s multipurpose solution.
Explore more products

For Astigmatism

Multifocal

Near/Far Sighted
Solutions
References
*Based on clinical studies with AIR OPTIXTM AQUA contact lenses.
**Based on clinical studies with AIR OPTIXTM AQUA contact lenses.
†Based on a clinical study with AIR OPTIXTM AQUA and AIR OPTIXTM NIGHT & DAYTM AQUA contact lenses.
††Demonstrated in AIR OPTIXTM plus HydraGlydeTM sphere lenses compared to AIR OPTIXTM AQUA sphere contact lenses.
‡Based on a clinical study with AIR OPTIXTM for Astigmatism contact lenses.
‡‡Astigmatic eyes are eyes with a spectacle cylinder of at least -0.75DC.
§Among symptomatic lens wearers, those who at baseline experienced end-of-day discomfort or had to remove their lenses earlier than they wished.
§§In separate studies, AIR OPTIXTM AQUA contact lenses were tested with habitual multipurpose solution followed by 30 days of use with either OPTI-FREETM PureMoistTM Multipurpose Disinfecting Solution or AOSEPTTM PLUS with HydraGlydeTM Cleaning & Disinfecting Solution. In both studies, comfortable wear time with habitual multipurpose solution was measured at baseline, and with study solution at Day 30.
∂Eye exam may be required. Professional fees may apply.
AOSEPTTM is a trademark of American Optical.
References: 1. Nash W, Gabriel M. Ex vivo analysis of cholesterol deposition for commercially available silicone hydrogel contact lenses using a fluorometric enzymatic assay. Eye Contact Lens. 2014;40(5):277-282. 2. Nash W, Gabriel M, Mowrey-Mckee M. A comparison of various silicone hydrogel lenses; lipid and protein deposition as a result of daily wear. Optom Vis Sci. 2010;87:E-abstract 105110. 3. Lemp J, Kern J. A comparison of real time and recall comfort assessments. Optom Vis Sci. 2016;93:E-abstract 165256. 4. Eiden SB, Davis R, Bergenske P. Prospective study of lotrafilcon B lenses comparing 2 versus 4 weeks of wear for objective and subjective measures of health, comfort and vision. Eye Contact Lens. 2013;39(4):290-294. 5. Luensmann D, Yu M, Yang J, Srinivasan S, Jones L. Impact of cosmetics on the physical dimension and optical performance of silicone hydrogel contact lenses. Eye Contact Lens. 2015;41(4):218-227. 6. Clinical assessment of a regimen of AIR OPTIXTM plus HydraGlydeTM silicone hydrogel lenses and HydraGlydeTM-containing lens care solutions; Alcon data on file, 2017.
7. In vitro study over 16 hours to measure wetting substantivity; Alcon data on file, 2015. 8. Lemp J, Kern J. On-eye performance of lotrafilcon B lenses packaged with a substantive wetting agent. Poster presented at Optometry’s Meeting, the Annual Meeting of the American Optometric Association; June 21-25, 2017; Washington, D.C. 9. In a randomized, subject-masked, multi-site clinical study with over 150 patients; significance demonstrated at the 0.05 level; Alcon data on file, 2005. 10. Luensmann D, Schaeffer JL, Rumney NJ, Stanberry A, Walsh K, Jones L. Spectacle prescriptions review to determine prevalence of ametropia and coverage of frequent replacement soft toric contact lenses. Cont Lens Anterior Eye. In press. 11. The 2013 Gallup Study of the U.S. Consumer Contact Lens Market. Syndicated research study conducted by Multi-Sponsor Surveys Inc. under a license agreement with Gallup Inc. 12. In a survey of 1,012 contact lens dropouts aged 35-50; Alcon data on file, 2013. 13. Garofalo R, Lemp J. Clinical trial experience with OPTI-FREETM PureMoistTM MPDS. Contact Lens Spectrum. Special edition. September 2011;44-48. 14. AIR OPTIXTM AQUA comfortable wear time comparison of day 30 to baseline. C-09-074 Additional analyses; Alcon data on file, 2011. 15. In a multicenter, prospective, bilateral eye, randomised, crossover, double masked (to brand) study comparing two one-step hydrogen peroxide lens care solutions in symptomatic contact lens wearers; Alcon data on file, 2016. 16. Lemp J, Guillon M, Wang C-H, Patel K, Gupta R, Patel A. Fitting success of lotrafilcon B lenses with different packaging solutions. Poster presented at British Contact Lens Association 2017 Clinical Conference and Exhibition; June 9-11, 2017; Liverpool, UK. 17. Internal memo from the designers of the PRECISION BALANCE™ 8|4 toric contact lens design; Alcon data on file, 2018. 18. Brobst A, Wang C-H, Rappon J. Clinical comparison of the visual performance of silicone hydrogel toric lenses with different stabilization systems. Cont Lens Anterior Eye. 2009;32(5):243.
See Brilliantly
Terms of Use
